The FiberCell Systems cartridge shown with tubing.
Shown in photo: Hybridoma cells growing at high density in the C2011 cartridge using CDM-HD medium
HFBRs: THE IDEAL SYSTEM FOR MONOCLONAL ANTIBODIES PRODUCTION FROM HYBRIDOMAS
When you fuse a B cell with a cancer cell to generate a hybridoma, you create a cell that inhibits its own growth. One of the ways that cancer cells locally immune suppress is by secreting a factor called TGFβ, which inhibits the growth and activity of B cells. By removing TGFβ while retaining IgG, you can greatly enhance the production of monoclonal antibodies in cell culture.
Based on this principle, HFBRs are the ideal system for the production of monoclonal antibodies from hybridoma cell lines as they allow TGFβ to diffuse away from the cells, while concentrating the antibody to high levels inside the hollow fiber bioreactor itself.
MOLECULAR WEIGHT CUT-OFF (MWCO) INFLUENCE ON CELL CONCENTRATION
The ability to control fiber molecular weight cut-off allows for the retention of higher concentrations of the desired products, controls cytokine effects, and facilitates the selective removal of inhibitory TGFβ. Hybridomas secrete an inhibitory factor called TGFβ, that in its active form, has a molecular weight of 27kD. If you remove the TGFβ while retaining the IgG, you can greatly enhance the production of monoclonal antibodies in cell culture. The MWCO of hollow fibers permits this inhibitory cytokine to diffuse away while the secreted antibody and cells remain in the small volume of the extra-capillary space.
The gross filtration rate of the polysulfone fiber is ten times higher than that of cellulosic fibers for more rapid exchange of nutrients and waste products. Cell densities of 108 or higher are attained by facilitating the reduction of serum, adapting to commercially available serum-free formulations and using FiberCell Systems’ CDM-HD.
Infographic on the passing of TGFβ through the hollow fiber while retaining the IgG in the ECS of the cartridge.

CDM-HD serum replacement bottle
HFBRs are an effective method for producing milligram to gram quantities of monoclonal antibodies and recombinant proteins. The harvested product is concentrated and free of contaminating proteins, DNA, RNA, and proteases. The use of CDM-HD renders the medium used, economical, chemically defined, and protein-free. Cultures can be maintained for long periods of time, and scalability of the system is determined by length of culture, not new equipment.
Learn more about FiberCell’s CDM-HD serum replacement for use with monoclonal antibodies here.
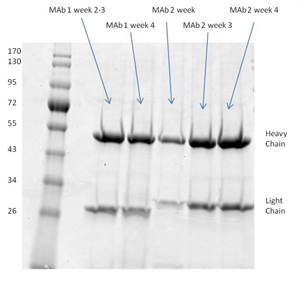


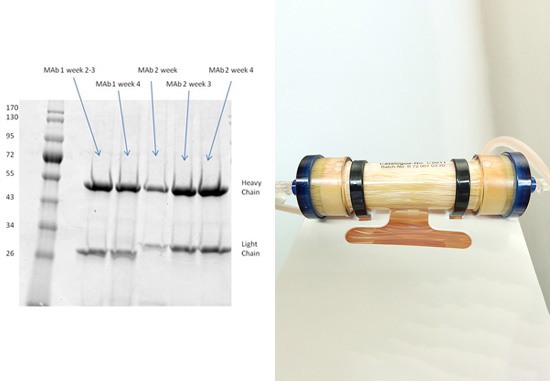
1. Higher concentration yield achieved using bioreacter with CDM-HD
Unpurified harvested supernatant showing high concentration of secreted monoclonal antibody produced using CDM-HD, as compared to using the more expensive FBS. See results here
2. SDS-page analysis of RAB M5114 supernatant
Clean harvests that are free from extraneous proteins were achieved using the FiberCell C2011 20 kD MWCO polysulfone fiber cartridge and DMEM with 10% CDM-HD medium. See results here
3. Direct comparison of C2011 fibercell cartridge and 20 liter wave™ bag
A direct comparison between a Wave bioreactor bag, standard T175 tissue culture flasks and a medium-sized FiberCell Systems C2011 hollow fiber bioreactor cartridge. See results here
4. C5011 cartridge specifically optimized for monoclonal antibody production
The C5011 is the same cartridge as the C2011 but with more than twice the oxygenation capacity and larger pump tubing for high flow rate. See results here