5 X 108 human adipose derived adult MSC were cultured in a FiberCell Systems HFBR for 8 weeks using DMEM/10% FBS in the circulating medium only, with basal DMEM in the ECS. 40 mL of supernatant were harvested weekly from the cartridge. To isolate EV, the collected serum-free conditioned medium was subjected to two steps of centrifugation 1) 3000 g, 20 mins to remove cell debris 2) 100,000 g to pellet extracellular vesicles. Size distribution and concentration of EVs were quantitated by tunable resistive pulse sensing (TRPS; (qNano, IZON Sciences). One interesting feature of this protocol is that the cells did not expand over this period of time as the glucose uptake rate remained constant. MSC that do not proliferate will also not differentiate. At the end of 8 weeks the cartridge was cut open and cells recovered for phenotypic analysis. This demonstrated no change in cell phenotype over the period of culture.
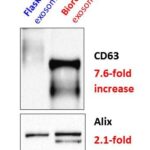
Comparison of flask culture to a C2011 HFBR for the production of extracellar vesicles from mesenchymal stem cells
| Collection Volume (mL) | Total Extra Vesicles Protein (mg) | Total Total Extra Vesicle Particles (1010) | ||||
| HFBR | ||||||
| Cartridge #1 (7 weeks, collection every week) | 240 | 11.82 | 95.78 | |||
| Cartridge #2 (4 weeks , 6 collections) | 120 | 14.45 | 326.9 | |||
| Flasks | ||||||
| 130 T225 | 4000 | 0.9 | 1.6 | |||
A flow cytometry dye transfer assay was used to demonstrate the ability of EV to deliver cargo to endothelial cells. A scratch closure test and rat skin wound healing assay was used to measure wound healing activity.
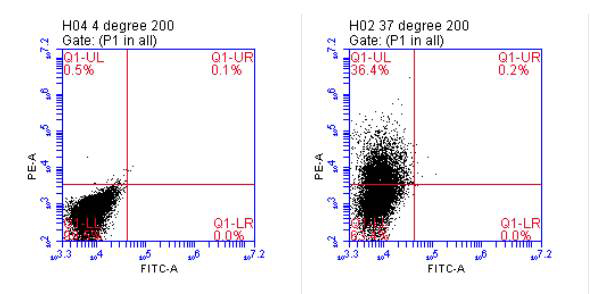
Incorporation of extracellular vesicles into human endothelial cells. In order for extracellular vesicles to transfer their cargo (nucleic acid, protein), they must somehow be incorporated into the recipient cell. Lipophilic dye transferred from extracellular vesicles and incorporated into cultured cells has been used to support the function of extracellular vesicles to deliver cargo into cells . We incubated Vybrant DiI cell labeling solution (Life Technologies) with approximately 5 x 108 for extracellular vesicles for 20 min at 37oC, followed by untracentrifugation at 100,000 xg for 2 hr to remove unincorporated dye. The labeled exosome pellet was resupended in PBS and added to cultured endothelial cells. Following incubation at 37oC for the experimental, and 4oC for the negative control, cells were removed from the plate by trypsinization and analyzed by flow cytometry. As shown by the shift in the population in the right panel, the cells incorporated DiI from the labeled extracellular vesicles , supporting the notion that these exosome preparations from the bioreactor are capable of delivering their cargo (i.e.: nucleic acid, protein) into the cell.
Wound healing in response to topical application of EVs. Left panel shows fresh 2 cm diameter wounds in the back of the rat. Right panel shows degree of wound healing after 19 days. Yellow circles mark vesicle control treatment. Red circles mark EV treated wounds. All treatments were single application. Pictures are illustrative of 9 different animals.
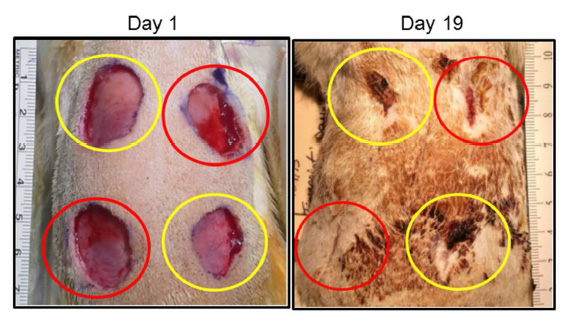
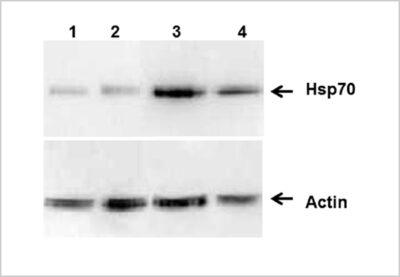
Figure A
Figure B
The use of the hollow fiber bioreactor allowed a uniform and consistent culture condition manipulation to be applied to a large number of cells, with ease.