We want to hear from you!
Feel free to share any feedback or suggestions you have for us to improve our products and/or services.
The Hollow Fiber Bioreactor (HFBR) is the best and one of the only methods that can model the mammalian circulatory system for in vivo-like conditions. Get a cleaner, simpler, and more cost-effective harvest with Fibercell Systems Hollow Fiber Bioreactors. Click here to find out more about the state-of-the-art technology behind the HFBR.
Of course! Low density suspension cultures or 2-D flask based processes, and the use of serum is inherently non-physiologic. While well-understood, robust, and convenient, classical batch style 2-D cultures are not biologically relevant systems and not the best way to culture cells. HFBRs provide a more physiologic, in vivo -like 3-D environment than other cell culture methods, and can also result in improved protein folding and more uniform post-translation modifications over time in a continuous, perfusion based process.
Find out more about harvesting recombinant proteins using HFBRs here.
Did not find the answer to your question here? Contact us to find out more.
Feel free to share any feedback or suggestions you have for us to improve our products and/or services.
Simply perform your harvest as usual. One of the issues that many researchers have when using our system is that they are afraid to harvest out too many cells. The cartridge can be overgrown if you don’t remove cells. After centrifugation your cell pellet should be between 1-3 mL or more (with the C5011 as much as 5 mL). Simply decant off the supernatant and re-suspend the cell pellet in 2 pellet volumes of fresh medium that has been diluted 10% with sterile distilled water. Leave it overnight at room temperature. The cells will consume the medium and also the hypotonicity will slightly squeeze the cells causing them to release any intracellularly sequestered antibody. This will get you an extra few milligrams every time you harvest.

Too few cells in relation to volume of media can result in lag phase. In the hollow fiber cartridge lag phase can be defined by the cells not consuming glucose but still show significant viability via the trypan exclusion assay. Always seed the cartridge with the recommended cell density. If this is not possible, adjust the volume of media down to keep the cells in culture from being too dilute.If the cells do go into lag phase you can either:

Hybridoma cells growing at high density in the C2011 cartridge using CDM-HD medium
To keep contamination at a minimum, clean the inside of the hood weekly, remembering to remove the working surface and clean underneath. Be sure the hood is validated on a regular basis. Never pour media, always pipette. Always wear a lab coat. Wait 60 seconds for the air currents to stabilize inside the incubator. Pull hair back and be sure to never have bare flesh inside the hood. Use plenty of alcohol and wipes but remember, it is not the application of alcohol that sterilizes, it is the evaporation. Good sterile technique will allow production from the cartridge for many months or more. We have produced a monoclonal antibody for over one year of continuous production.
New cell lines should always be quarantined until a mycoplasma screen has shown negative. The presence of mycoplasma contamination can be hard to detect without specific assays. The culture will just appear to be non-productive.
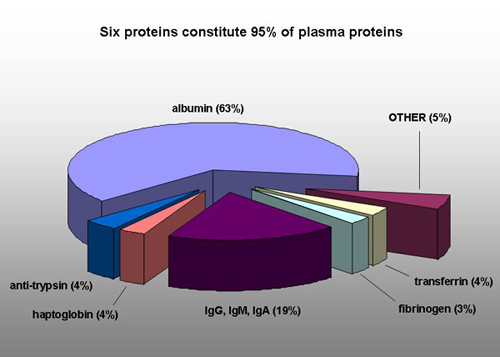
Various components of serum. The major component, BSA binds many substances non-specifically and can be an overwhelming protein burden in some purification schemes, immunoglobulins can interfere with specific antibody purifications and the “other” can have many undefined effects. None of these characteristics will be reproducible from lot to lot.