An HEK 293 culture expressing heterodimeric IL-15 was maintained in a hollow fiber bioreactor for over 4 months of continuous production, with 3 harvests of 20 mL per week.
The HEK 293 bioreactor culture yielded an equivalent number of exosomes per harvest as 70 T225 flasks. CD63 and Alix were greatly enriched from the bioreactor compared to flask culture. EV/protein ratio was 10-fold higher in harvests from the bioreactor suggesting a significant reduction in contaminating cell membrane fragments. Purified HEK 293 cells retained their IL-15 biological activity.
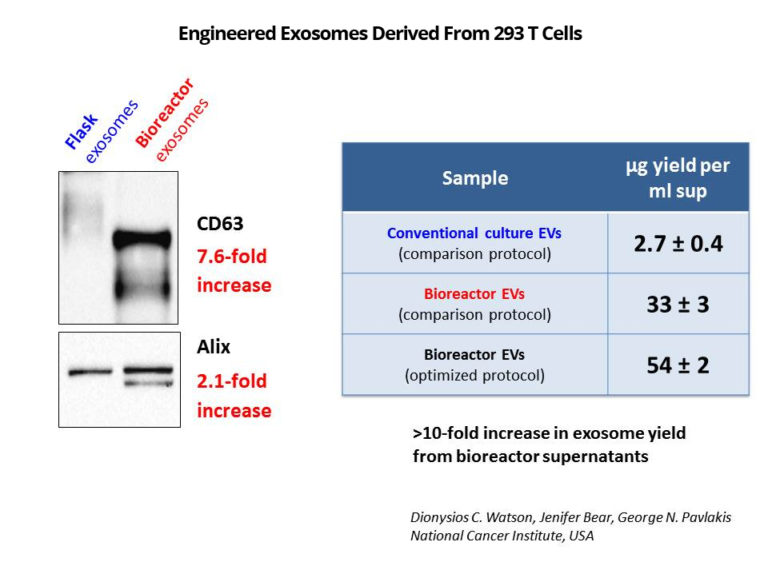
“We developed a methodology for the mass production of highly purified, bioactive EV using a lab-scale hollow fiber bioreactor, which may facilitate further in vivo studies. This method presents many advantages over conventional culture that likely contribute to the higher EV production yield and purity that we observed. First, lab-scale hollow fiber bioreactors enable the sustained maintenance of large numbers of cells (estimated by the manufacturer to be in the order of 109 cells) within a standard incubator, with minimal maintenance. More importantly, all of the cultured cells secrete EV into the relatively small volume of the culture cartridge extracapillary space (~20 mL) resulting in EV-rich conditioned medium that can be used directly for EV purification. Our findings suggest that this method yields 40-fold more EV particles per volume of conditioned medium vs. conventional cell culture. Thus, to match a single daily harvest of the hollow-fiber bioreactor (20 mL), an estimated 53 large (175 cm2) flasks producing 800 mL conditioned medium would be required. Moreover, cells grown in hollow-fiber bioreactors readily adapt to sustained growth in protein-free medium, which also facilitates EV purification without serum contaminants, as highlighted by our comparative proteomics study. On the other hand, bioreactor EV preparations may have a more diverse population of EV, as suggested by the increased size range. Thus, further purification of preparations (e.g. by density gradient) may be required for some applications.
Our findings showing the greatly enhanced yield of EV purified from hollow-fiber cultures may also have significant implications for the development of clinical grade EV therapeutics, given that hollow-fiber methods have already been used to expand primary human cells under cGMP conditions. “*
*Efficient production and enhanced tumor delivery of engineered extracellular vesicles Watson, DC et al, Biomaterials 105 (2016) 195-206